

The Heisenberg uncertainty principle says that determining a particle’s position and velocity at the same time is impossible. Hence, the uncertainty in the momentum of the electron is 5.28 x 10 – ² 5 kg m s -1 Conclusion The second reason an electron cannot exist inside the nucleus is that no electron or particle in the atom has an energy greater than 4 MeV, according to experimental evidence.Īs a result, it has been established that electrons do not exist within the nucleus.Įxample 2: Calculate the uncertainty in the momentum of an electron if uncertainty in its position is 1 Å ( 10 -10 m).Īccording to Heisenberg’s uncertainty principle: However, beta particles (electrons) released from the nucleus during b-decay have an energy of around 3 MeV, which differs significantly from the value obtained of 19.6 MeV. As a result, the following relativistic formula should be used to compute its energy:Į = √Īs a result, if the electron occurs in the nucleus, its energy should be in the range of 19.6 MeV. With such a large momentum, an electron’s velocity must be similar to that of light. If the uncertainty in the electron’s momentum is this, then the electron’s momentum should be at least of this order, p = 1.05×10-20 kg m/sec. If the electron is to exist inside the nucleus, the position of the electron must be uncertain.Īccording to the principle of uncertainty, The diameter of the nucleus is about 10-14 metres. Questions and solved answers for Heisenberg’s uncertainty principleĮxample 1: Let us suppose, for the sake of argument, that electrons exist in the nucleus. When position or momentum are accurately measured, it immediately suggests a higher inaccuracy in the measurement of the other quantity. Heisenberg’s uncertainty principle formula can also be expressed as: The uncertainty in position is denoted by the letter ∆x. The uncertainty in momentum is denoted by the letter ∆p. The Planck constant (6.62607004 x 10 -34 kg m 2 / s) is represented by h. As a result, this product of uncertainty will only be meaningful for atoms and subatomic particles with extremely small masses.Īt all times, the value of position and momentum is higher than h/4π. The product of position and velocity uncertainty is equal to or larger than a very small physical quantity, h. This principle states that each particle’s position and momentum cannot be measured with infinitely high precision at the same time. Werner Heisenberg, a German scientist, proposed this idea in 1927. Let us learn more about this with numerical questions on Heisenberg’s uncertainty principle and their answers in detail. The Heisenberg uncertainty principle is a basic principle of quantum physics that explains why a scientist cannot simultaneously measure many quantum variables.
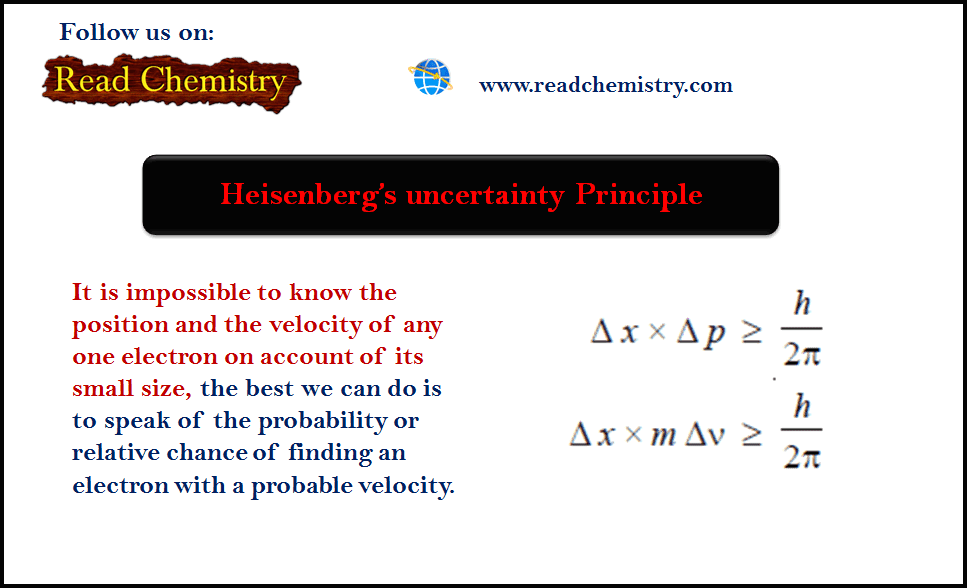

This is in contrast to classical Newtonian physics, which states that with good enough equipment, all particle variables are traceable to an unlimited uncertainty. The principle, which is commonly applied to a particle’s position and momentum, states that the more specifically a position is known, the more uncertain the momentum is, and the same happens when the momentum is known precisely and the position is uncertain. According to Heisenberg’s uncertainty principle, there is uncertainty while measuring a particle’s variable.


 0 kommentar(er)
0 kommentar(er)
